In this way, innovative platforms are created for biological applications with a focus on tissue engineering and biomaterial design.
A lab fully dedicated to soft lithography hosts all instruments required for master fabrication: chemical cabinet, spin coater, hot plates. A vacuum chamber, an oven, and a plasma cleaner enable the preparation and final sealing of the microfluidic device.
A second lab is equipped with cutting-edge instrumentation for rapid prototyping fabrication. In particular, the facility offers two fused deposition modeling 3D printers (Creality CR-30 and Prusa MK3RS) to produce objects with millimetric sizes, and one stereolithographic 3D printer (XFAB) enabling the design of devices with micrometric resolution.
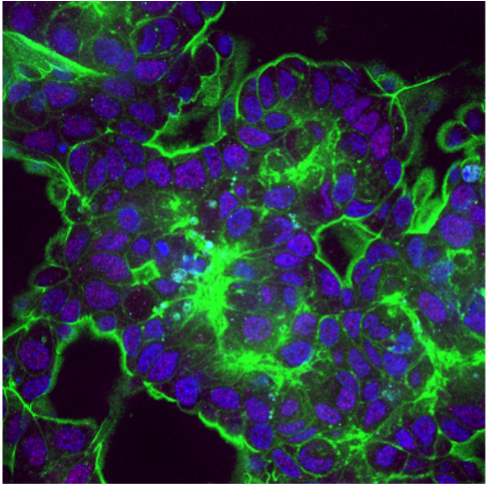
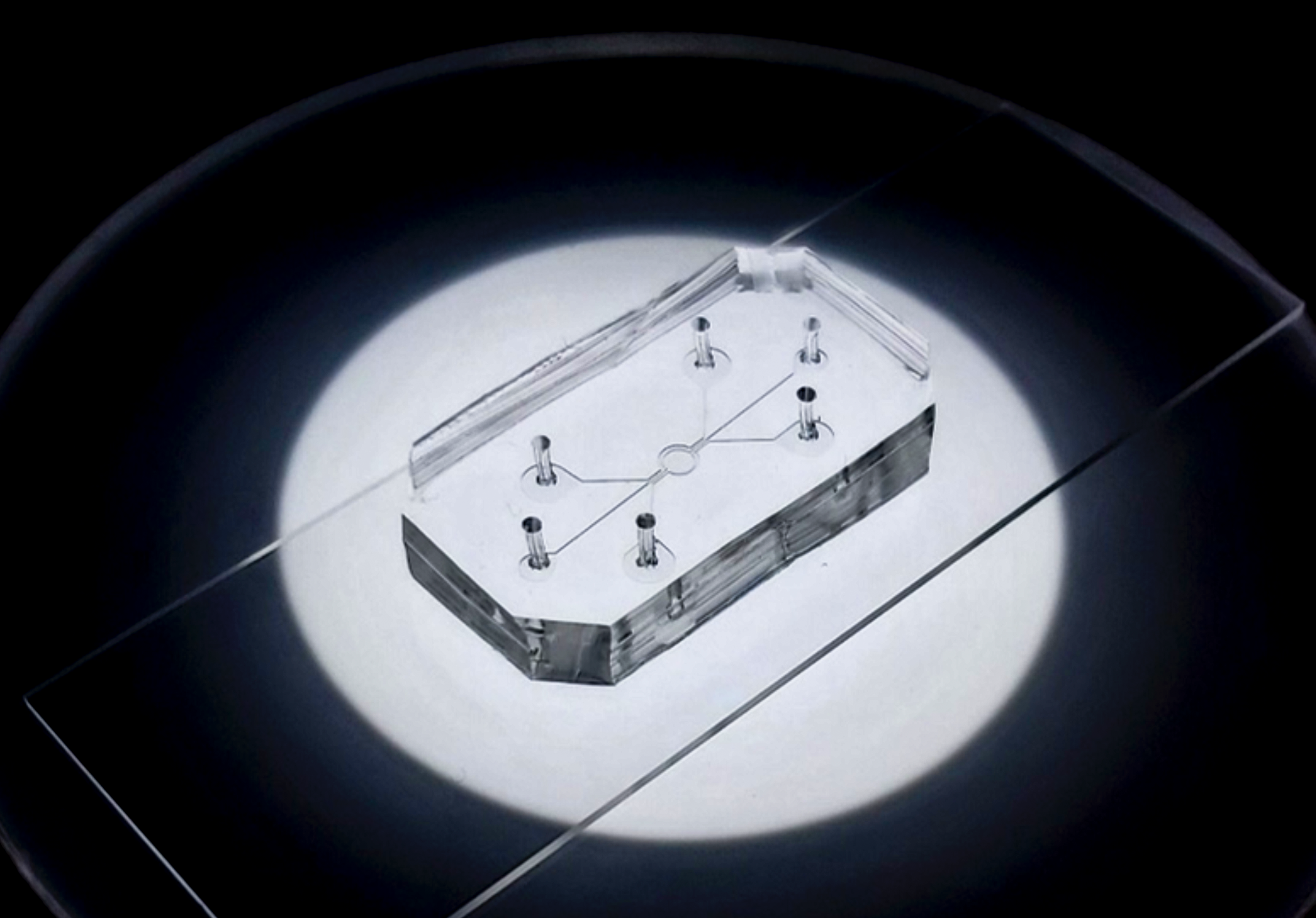
To date, the controlled spatial arrangement of multiple cell types of ovarian tumour microenvironment, i.e. cancer and immune cells, in a 3D niche is still unexplored. A 3D ovarian cancer (OCa) model is realised harnessing a 3D microfluidic platform. A 3D human OCa tissue is cultured in a circular microfluidic chamber, in communication with a microfluidic channel through a porous membrane. The latter is perfused with immune cells to simulate and study the dynamics of cell extravasation from the blood vessel and infiltration to the tissue. The effect of a potential novel therapeutic tool is investigated, quantitatively assessing multiple processes involved in tumour growth and immunogenicity.
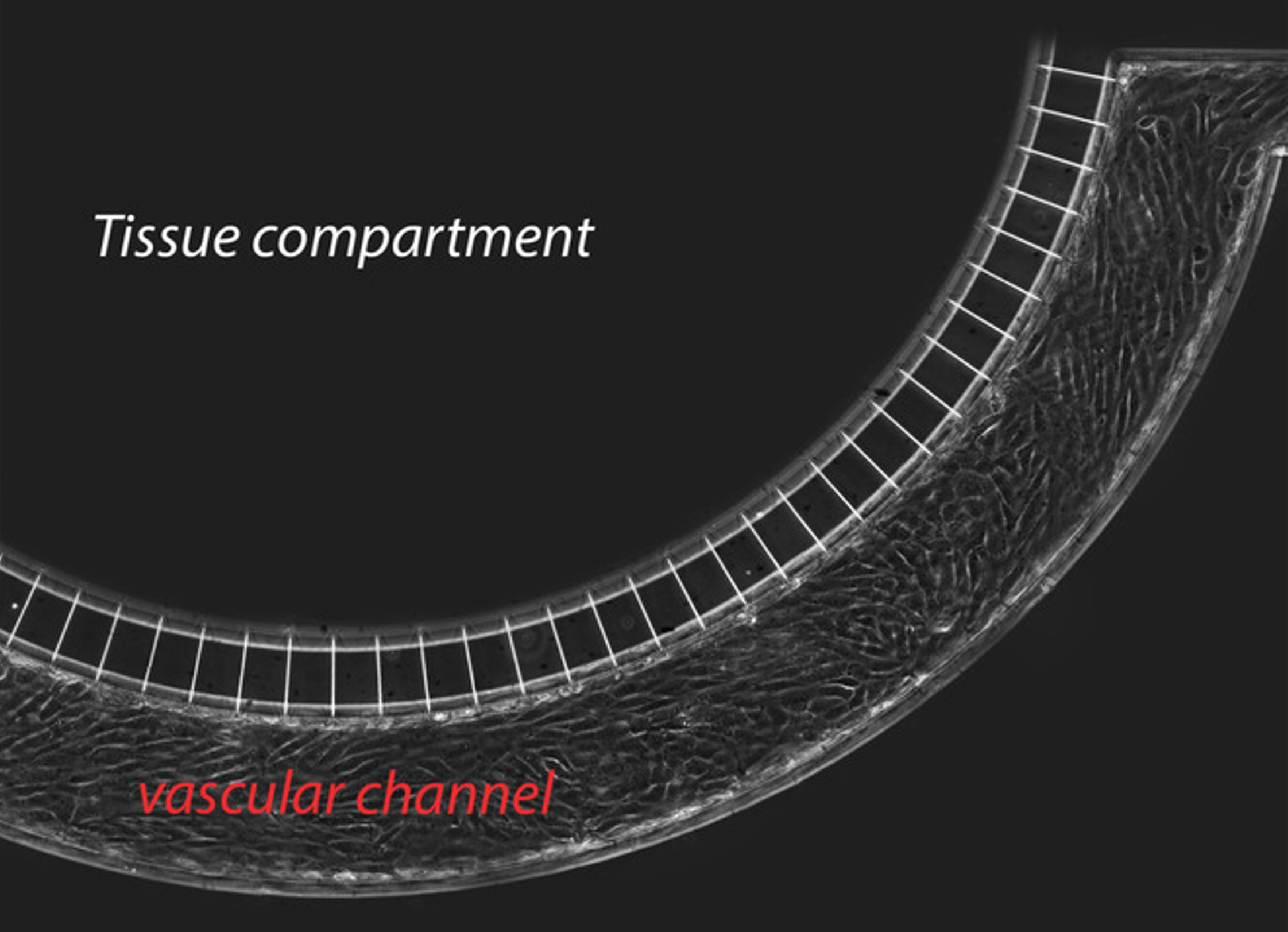
A local uptake of drugs at the target requires drugs diffusion across the endothelial barrier lining the blood vessel walls and through the surrounding tissue. A vessel-on-a-chip is exploited to investigate and quantify the effect of ultrasound-excited microbubbles -a process named cavitation- on endothelial integrity. Microbubbles amplify the ultrasound effect, leading to the formation of inter-endothelial gaps that cause barrier permeabilization and enhance drug delivery. The proposed integrated platform allows for precise and repeatable in vitro measurements of cavitation-enhanced endothelium permeability and shows potential for validating irradiation protocols for in vivo applications
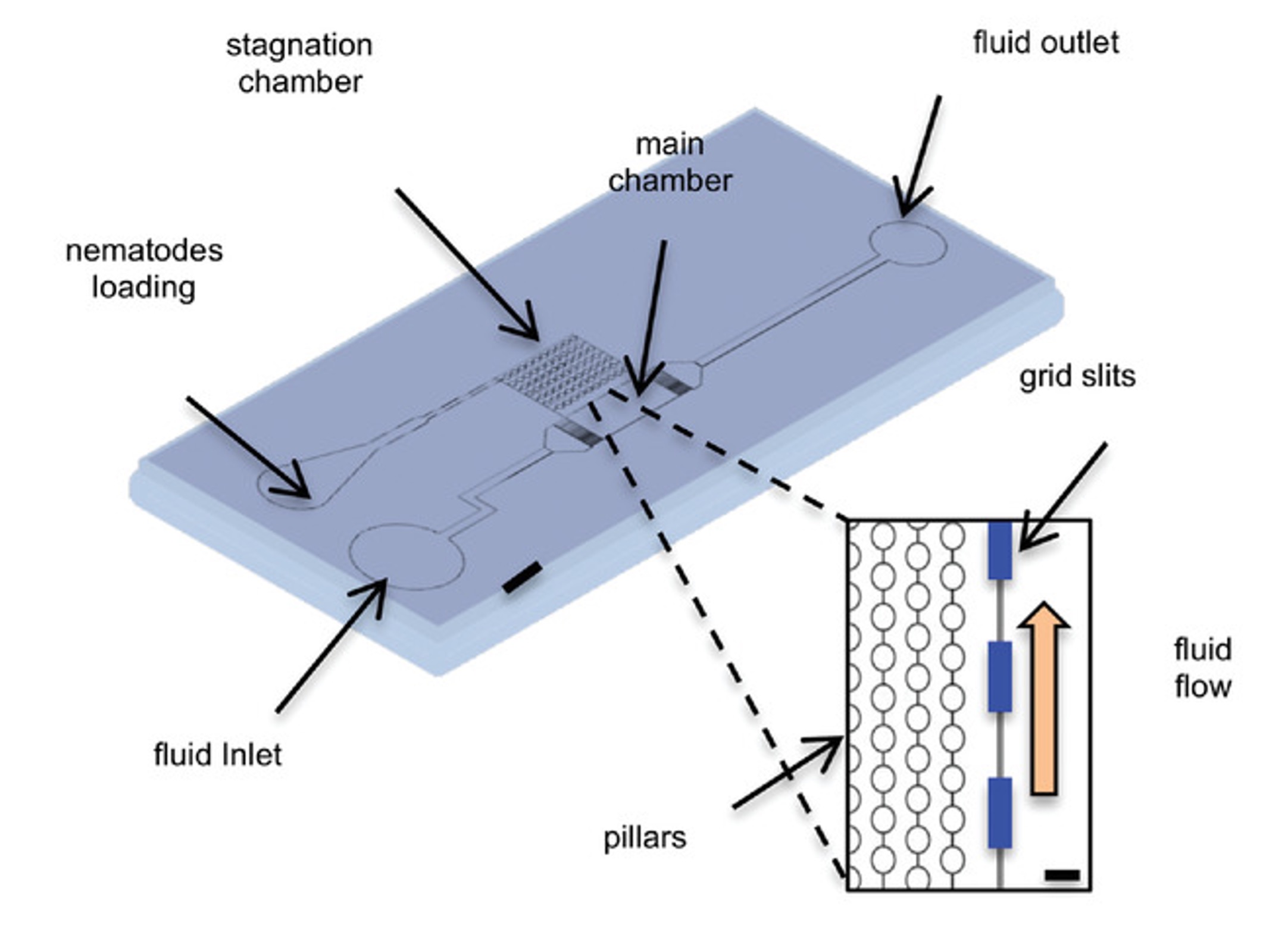
The AWC olfactory neurons play a key role in directing chemotaxis towards airborne attractants in Caenorhabditis elegans. This study demonstrates that AWCon neurons react to not only chemical signals but also mechanical disturbances, such as alterations in fluid flow within a microfluidic apparatus. A newly developed microfluidic device, aimed at reducing fluid shear flow, was used to further explore the previously unobserved mechanosensitivity of AWCon neurons. Nematodes within this device displayed a decrease in AWCon neuronal activity, suggesting that the tangential aspect of mechanical stress predominantly contributes to AWCon's mechanosensitivity. Moreover, this microfluidic system, which combines shearless perfusion with calcium imaging, offers an innovative and more precise method for in vivo studies in both micro-organisms and cultured cells.
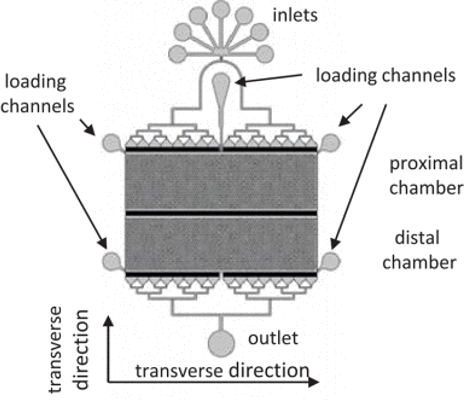
Calcium imaging studies using C. elegans are gaining popularity due to the nematode's benefits in fields from biology to neuroscience. Microfluidics plays a crucial role, offering controlled environments and enhancing experiment throughput. In this context, we introduce a new device that allows for calcium imaging with precise, timed delivery of chemical stimuli to distinct groups of nematodes. By using barriers to separate the worms, the device simplifies linking specific responses to different strains, aiding in experiment parallelization and increasing throughput. This innovation could pave the way for developing a biosensor based on C. elegans' remarkable sensory capabilities.
